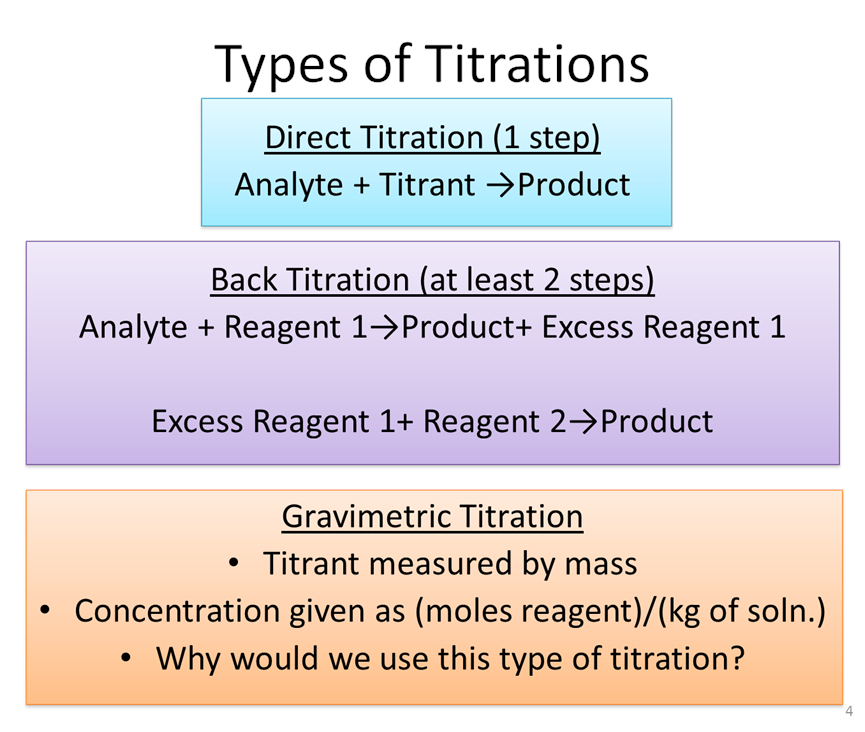
Define Titration With Example . titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Learn the titration graph and titration equation. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What are titrant and analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.
from chem.libretexts.org
titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn the titration graph and titration equation. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration. What are titrant and analyte. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the.
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts
Define Titration With Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What are titrant and analyte. Learn the titration graph and titration equation. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration.
From slideplayer.com
Outline Overview Terminology and Definitions Basic Concept ppt download Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What are titrant and analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Learn the titration graph and. Define Titration With Example.
From www.slideserve.com
PPT Precipitation Titration PowerPoint Presentation ID3571749 Define Titration With Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What is the equivalence point of a titration. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Learn. Define Titration With Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What are titrant and analyte. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the slow addition of one solution. Define Titration With Example.
From www.slideshare.net
Complexometric titrations Define Titration With Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an.. Define Titration With Example.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax General Chemistry Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration refers to a process where the. Define Titration With Example.
From www.science-revision.co.uk
Titrations Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the slow addition of one solution of a known. Define Titration With Example.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Education Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory. Define Titration With Example.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Define Titration With Example What is the equivalence point of a titration. Learn the titration graph and titration equation. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the. Define Titration With Example.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Define Titration With Example What is the equivalence point of a titration. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Learn the titration graph and titration equation. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Define Titration With Example.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID2144898 Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration (also known as titrimetry[1] and volumetric analysis) is. Define Titration With Example.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Define Titration With Example What are titrant and analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution is. Define Titration With Example.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Define Titration With Example What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Learn the titration graph and titration equation. titration is the process in which one solution is added to another solution such that it reacts under conditions. Define Titration With Example.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Define Titration With Example Learn the titration graph and titration equation. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration (also known as titrimetry[1]. Define Titration With Example.
From www.youtube.com
Chemistry & Biology Define the Term Titration YouTube Define Titration With Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration (also known as titrimetry[1] and volumetric analysis) is. Define Titration With Example.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What is the equivalence point of a titration. titration is the process in. Define Titration With Example.
From exoordqpq.blob.core.windows.net
Titrate Meaning Chemistry at Elfriede Downey blog Define Titration With Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Learn the titration graph and titration equation. What is the equivalence point. Define Titration With Example.
From theedge.com.hk
Chemistry How To Titration The Edge Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What are titrant and analyte. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution. Define Titration With Example.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration, process of chemical analysis in which the quantity of some constituent of. Define Titration With Example.